Treating HRS-AKI
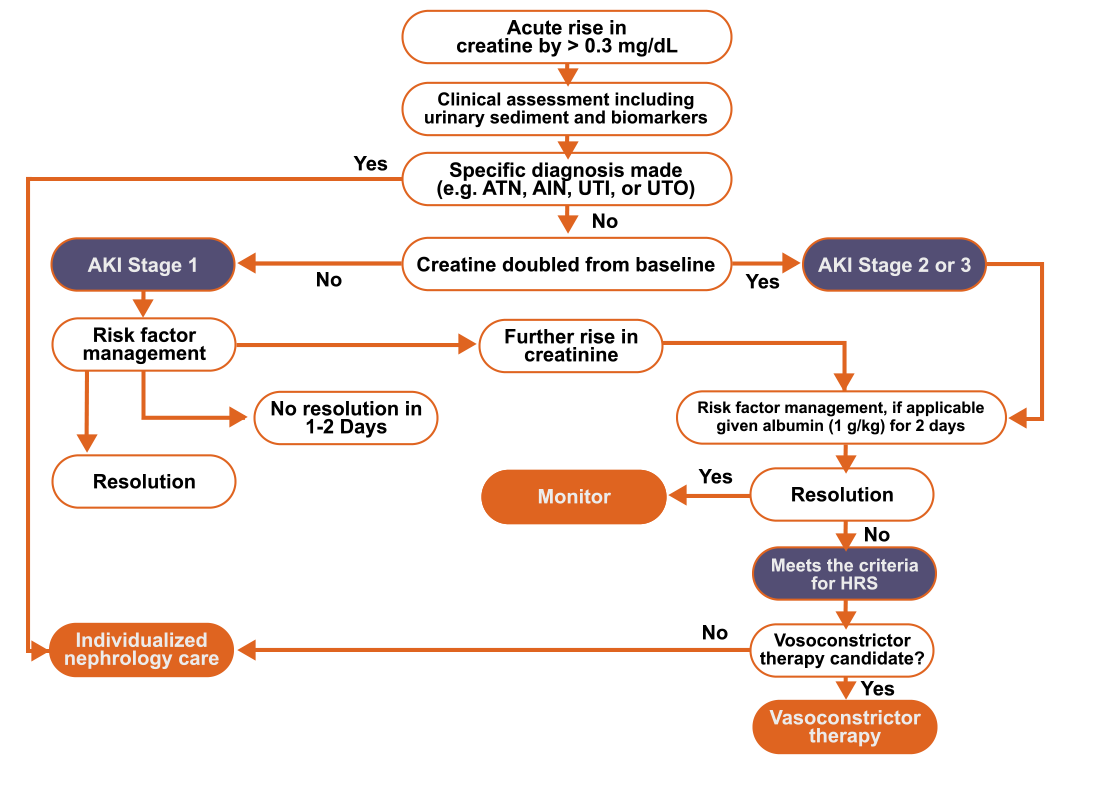
HRS, hepatorenal syndrome
Vasoconstrictors, in combination with albumin, are effective in improving kidney function in patients with HRS-AKI. The vasoconstrictor of choice for HRS-AKI is terlipressin. In settings where terlipressin is not available, norepinephrine should be considered, typically in an intensive care setting. If neither can be administered, a trial of oral midodrine in combination with octreotide may be considered, but its efficacy is low. All vasoconstrictors should be dosed according to the table; albumin 25% should be administered in combination with each vasoconstrictor and dosed according to clinical parameters. Response to vasoconstrictor therapy is defined by SCr decreasing to < 1.5 mg/dL or returning to within 0.3 mg/dL of baseline over a maximum of 14 days. In patients whose SCr remains at or above the pretreatment level over 4 days with the maximum tolerated doses of the vasoconstrictor, therapy may be discontinued. Disease may recur after discontinuation of treatment, so close follow-up is warranted. If there is recurrence, patients should be retreated.
Patients with HRS-AKI have a better response to therapy if therapy is started earlier rather than later. Terlipressin, when available, should be considered the treatment of choice as response rates are better than the poor response rates of midodrine, octreotide, and albumin.
*Terlipressin is currently an investigational agent being evaluated for the treatment of HRS in the U.S., and its safety and effectiveness have not yet been established by the FDA.
AKI, acute kidney injury; HRS, hepatorenal syndrome
Patients on vasoconstrictors and albumin should be closely monitored for the possible development of adverse effects. Adverse effects are infrequent, are mainly related to vasoconstrictors, and include hypertension, peripheral ischemia, abdominal pain, nausea, diarrhea, headache, intestinal ischemia, and cardiac ischemia. Pulmonary edema may develop from fluid overload related to albumin infusion. Early intervention may prevent more serious consequences, and most resolve after dose reduction or discontinuation of therapy.
Additional Special Considerations in HRS Management
AKI, acute kidney injury; HRS, hepatorenal syndrome